What are diamond and graphite in relation to carbon?
They're both carbon allotropes, however they are arranged differently. Diamond and graphite are both allotropes of carbon. Allotropes are basically different forms of the same element. The only difference is the structure and arrangement of how the carbon atoms are oriented. As you can see, graphite is arranged in a sheet-like arrangement and when used in pencils, sheets of graphite are removed when writing. As for diamonds, they are arranged in a geometric, 3D shape. This is the reason why they are considered the hardest, natural compound. Hope this helps :)
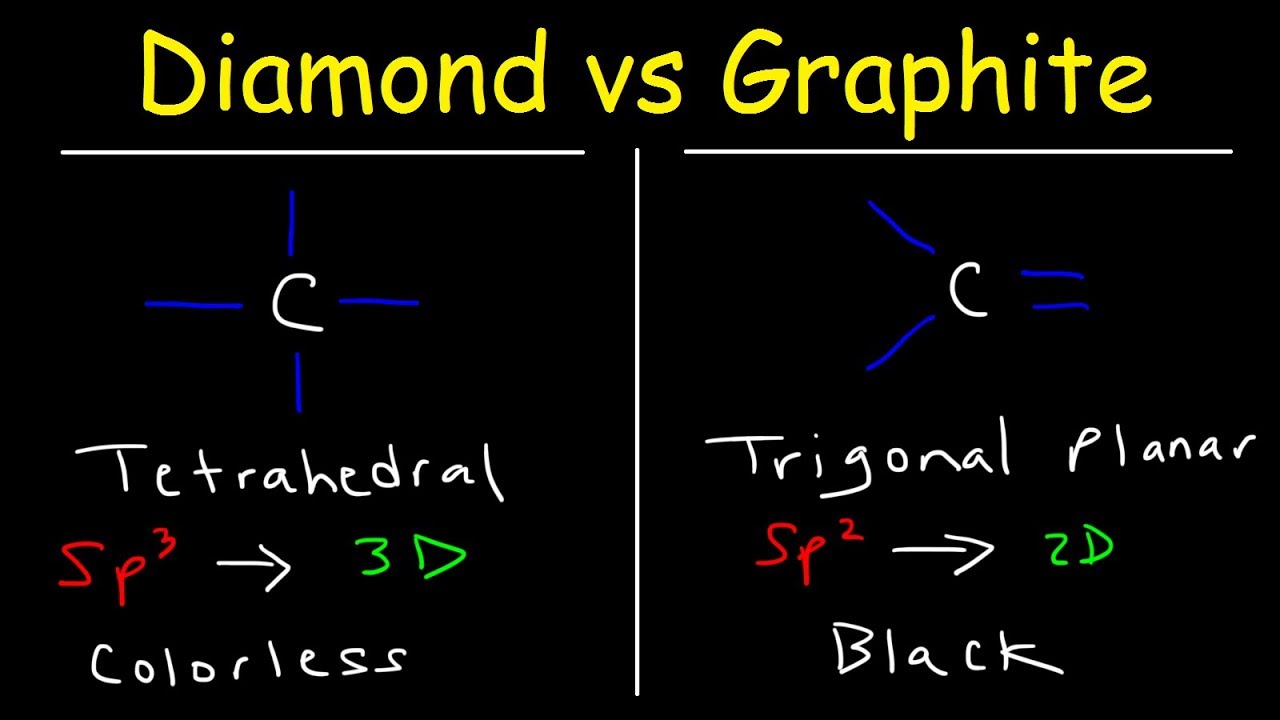
Structure of Diamond and Graphite, Properties - Basic Introduction

Turning graphite into diamond

A concise review of the Raman spectra of carbon allotropes
Diamond and graphite are two allotropes of carbon. What is an

Difference between diamond and graphite is due to

difference between diamond and graphite#allotrope of carbon
14.4A: Graphite and Diamond - Structure and Properties - Chemistry

Siyuan Yue - Difference of Graphite and Diamond

What Is an Allotrope? Definition and Examples in Chemistry
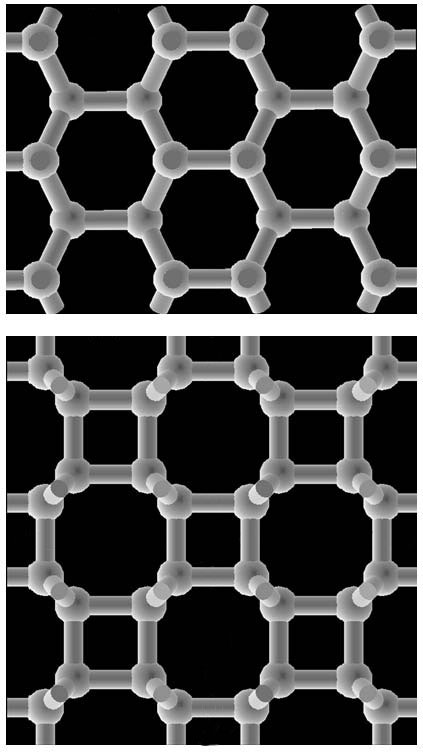
Physics - Between Graphite and Diamond
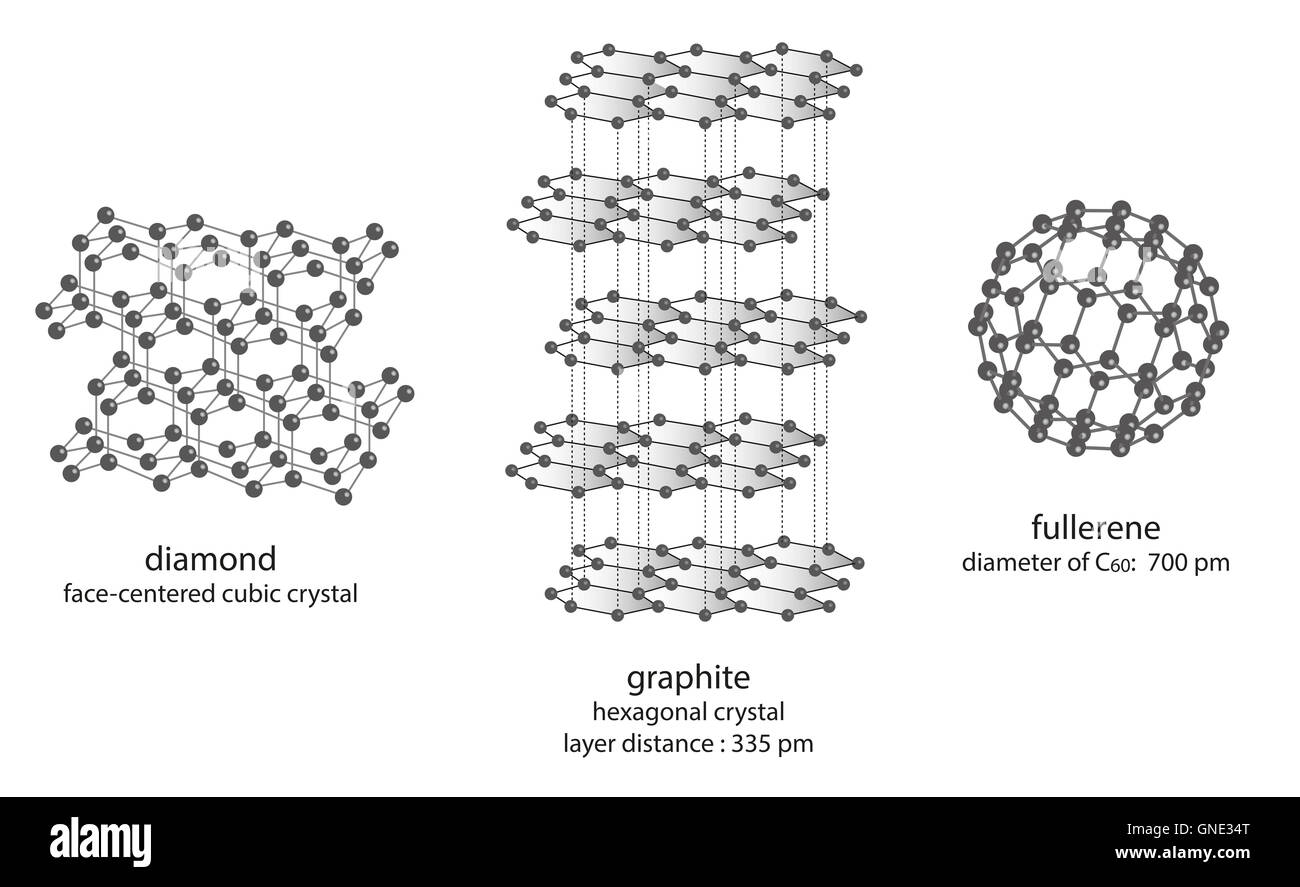
modification of carbon - molecule structure of diamond, graphite

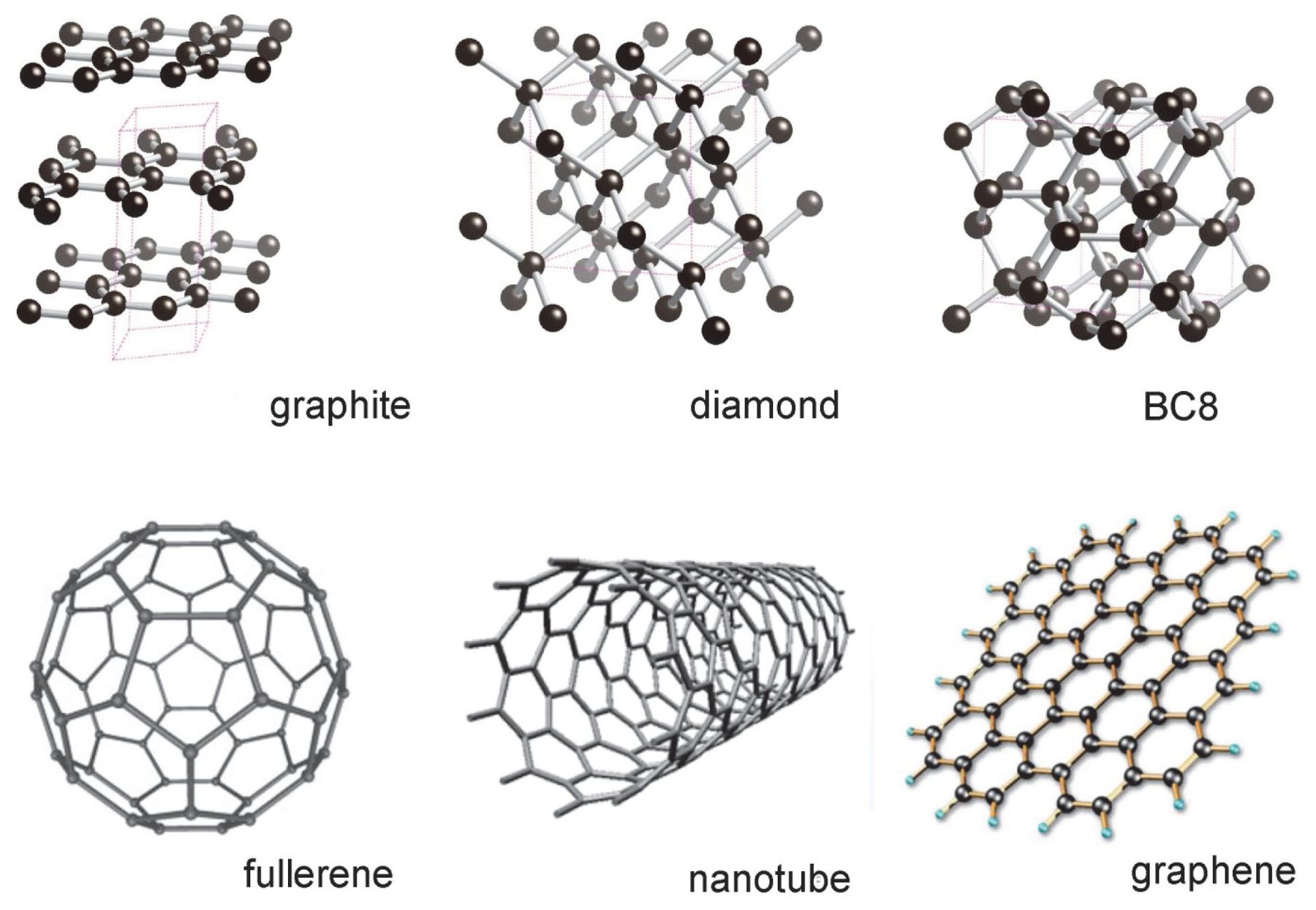
Carbon allotropes (diamond, graphite, graphene, types of coal, etc

Graphite Graphite is made up of carbon atoms. Chemical formula: C
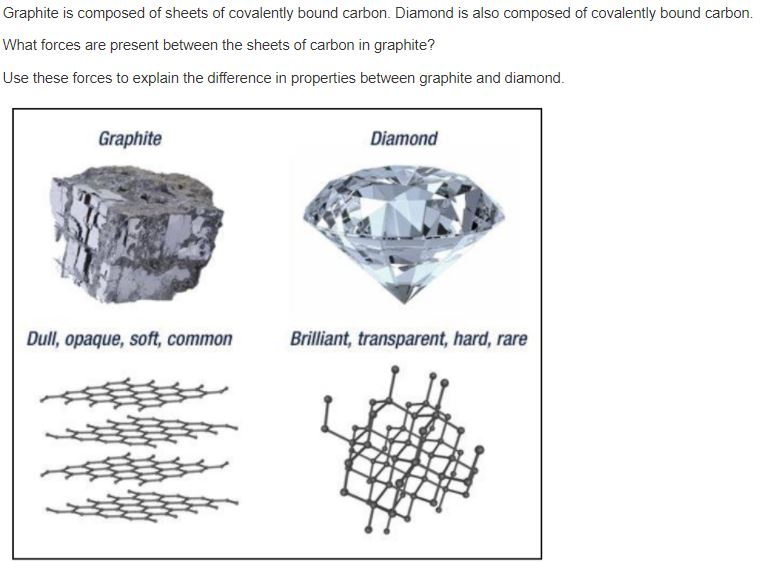
Solved Graphite is composed of sheets of covalently bound
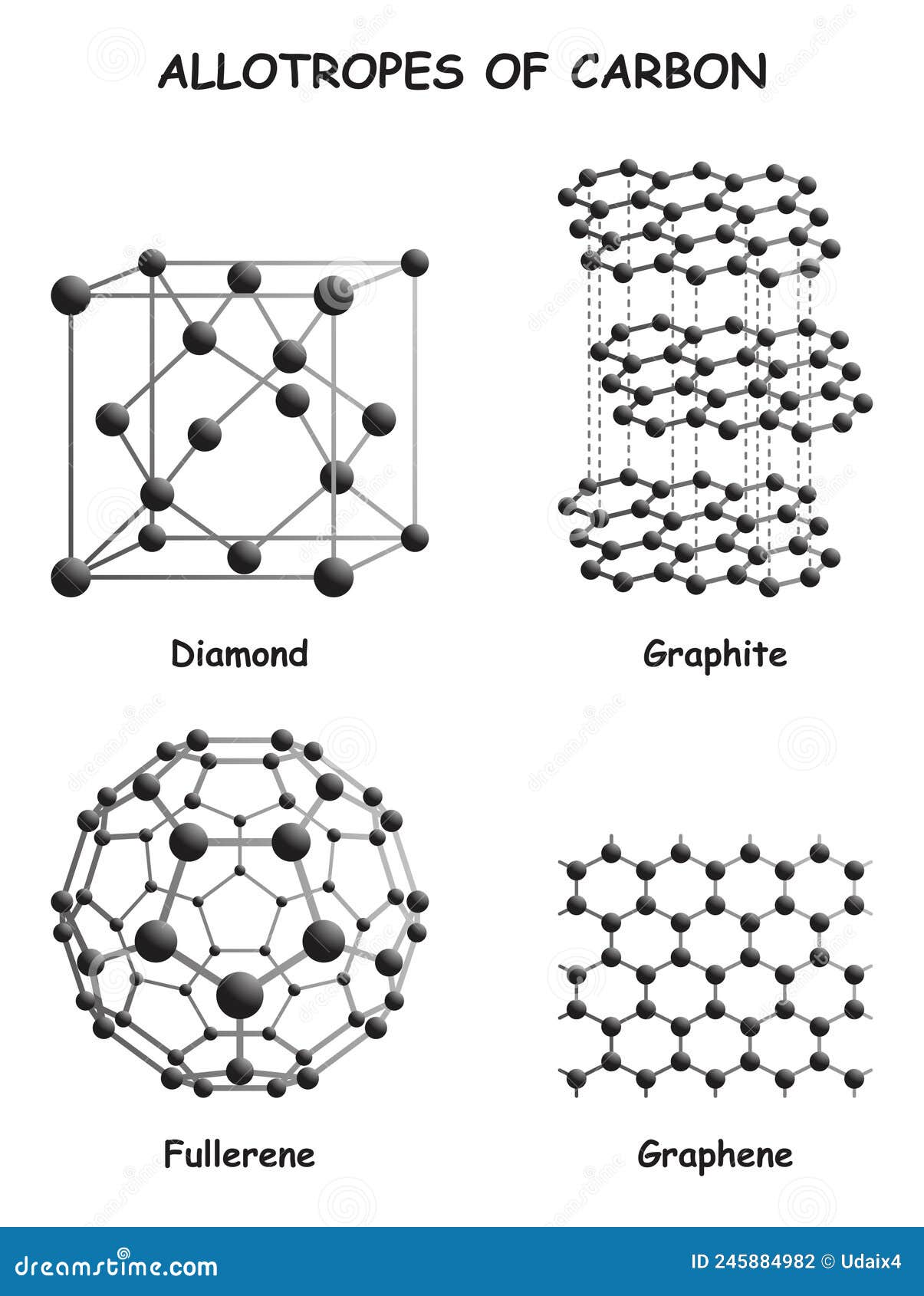
Allotropes of Carbon Infographic Diagram Stock Vector









