
For calcium carbonate draw both the cation and the anions as standalone ions. Draw the most common Lewis structure, and do not draw alternative resonance forms.
Molecules, Free Full-Text
Solved Se See Periodic Table Feedback (1 point) Part 1 For
Solved Part 3 (2 points) BagP2 There are two correct
Solved Ques on (2 points) Antacid tablets commonly contain

Formal Charges and Resonance
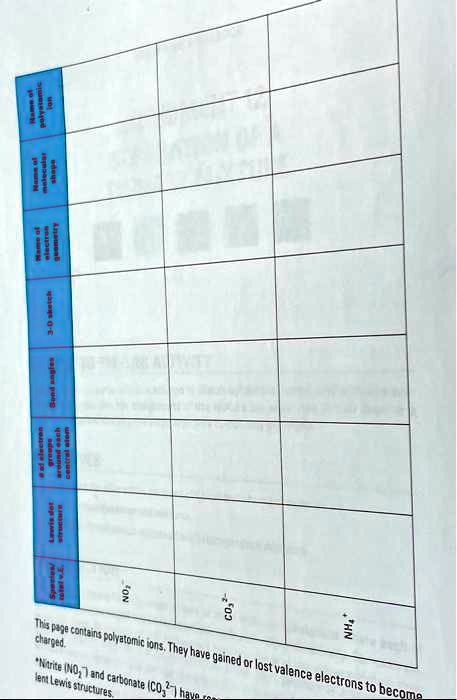
SOLVED: Please help. Page - 203 charged. This page contains polyatomic ions. They have gained or lost valence electrons to form NO3- and carbonate CO3^2-. Lewis structures are used to represent these ions.

What is the shape of the carbonate ion, (CO3)^2 ?
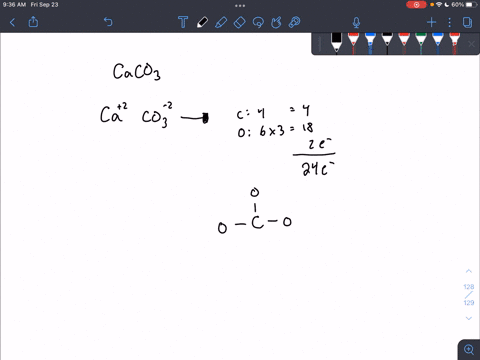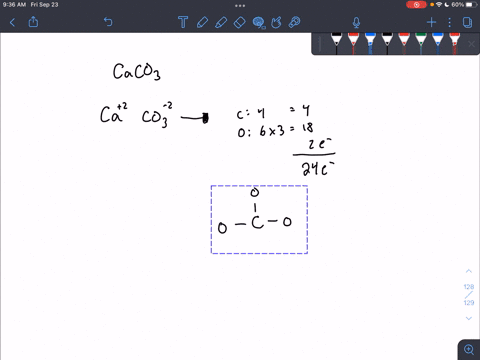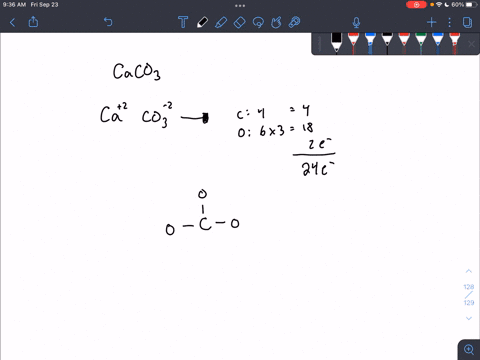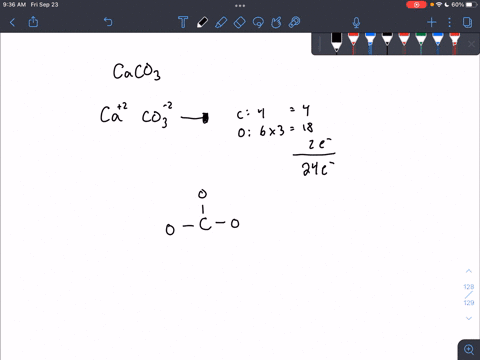
SOLVED: For each part draw both the the cation and the anions as standalone ions. Add formal charges on the appropriate atom. For each organic ion, draw just the most common Lewis

Draw all significantly contributing resonance structures for the

Draw the Lewis structure for the carbonate ion, CO32-. How many resonance forms are possible? Draw them.

Oxidation of the cyanide ion produces the stable cyanate ion (OCN









