Polymorph screening in pharmaceutical development - European Pharmaceutical Review
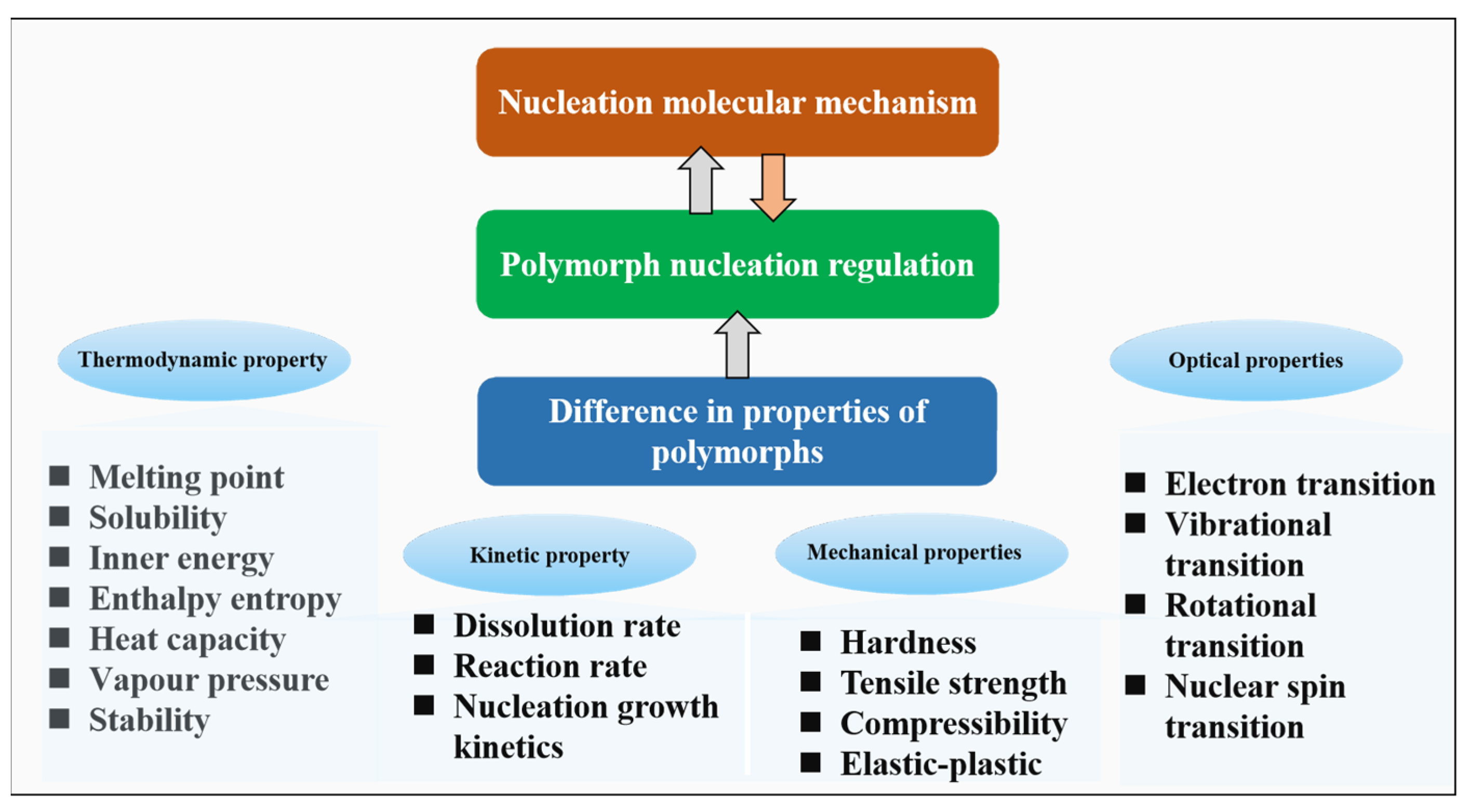
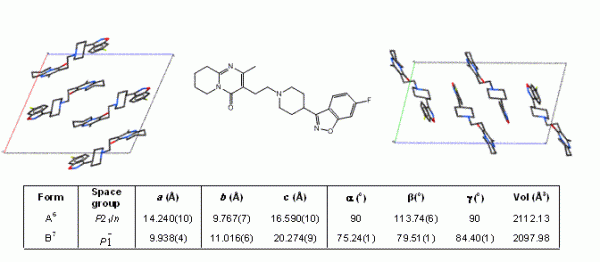
The majority of active pharmaceutical ingredients (APIs) are produced by crystallisation and so the phenomenon of polymorphism, whereby an organic molecule can adopt more than one crystalline form (Figure 1), is of considerable importance when trying to achieve consistent product quality during the manufacture of pharmaceutical solids and solid dosage forms. Although morphology and particle size-distribution are important solid-state characteristics, the uncontrolled occurrence of multiple physical forms (polymorphs, solvates, salts, co-crystals or amorphous) of an API can have significant effects on the performance of the material during processing, manufacture, storage and administration. For example, the solubility difference between some polymorphs has been shown to be over four times that of the least soluble form1 and can vary by significantly more for amorphous forms2.

Pharmaceutical Polymorphism Screening & Selection

WO2021032959A1 - Crystalline forms of pyrimidino diazepine derivative - Google Patents

A critical review on thermodynamic and hydrodynamic modeling and

What are Polymorphs?

Recent Advances in Polymorph Discovery Methods of Organic Crystals
Towards precision medicine: interrogating the human genome to

A practical guide to pharmaceutical polymorph screening

PDF) A Practical Guide to Pharmaceutical Polymorph Screening
Polymorph screening in pharmaceutical development - European

Solid-state and particle size control of pharmaceutical cocrystals
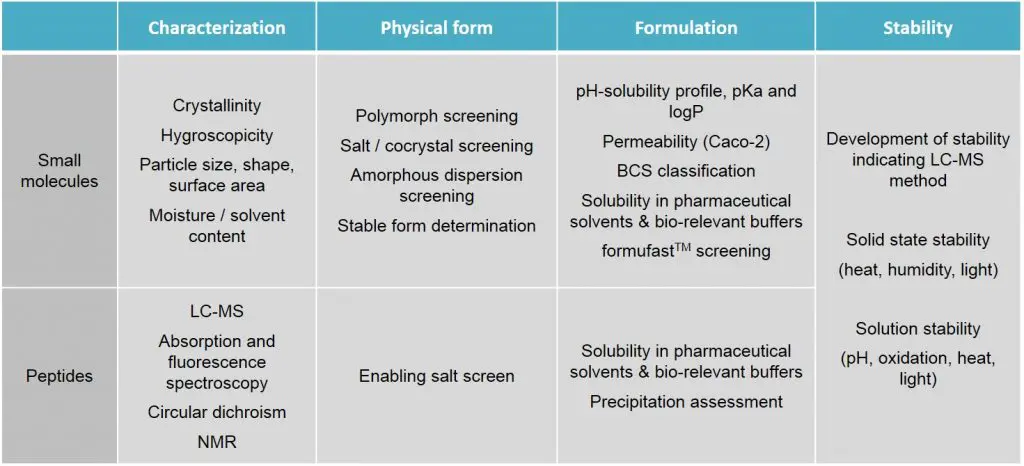
Preformulation - Almac
Combined crystal structure prediction and high-pressure

A practical guide to pharmaceutical polymorph screening

Pharmaceutical Crystallization