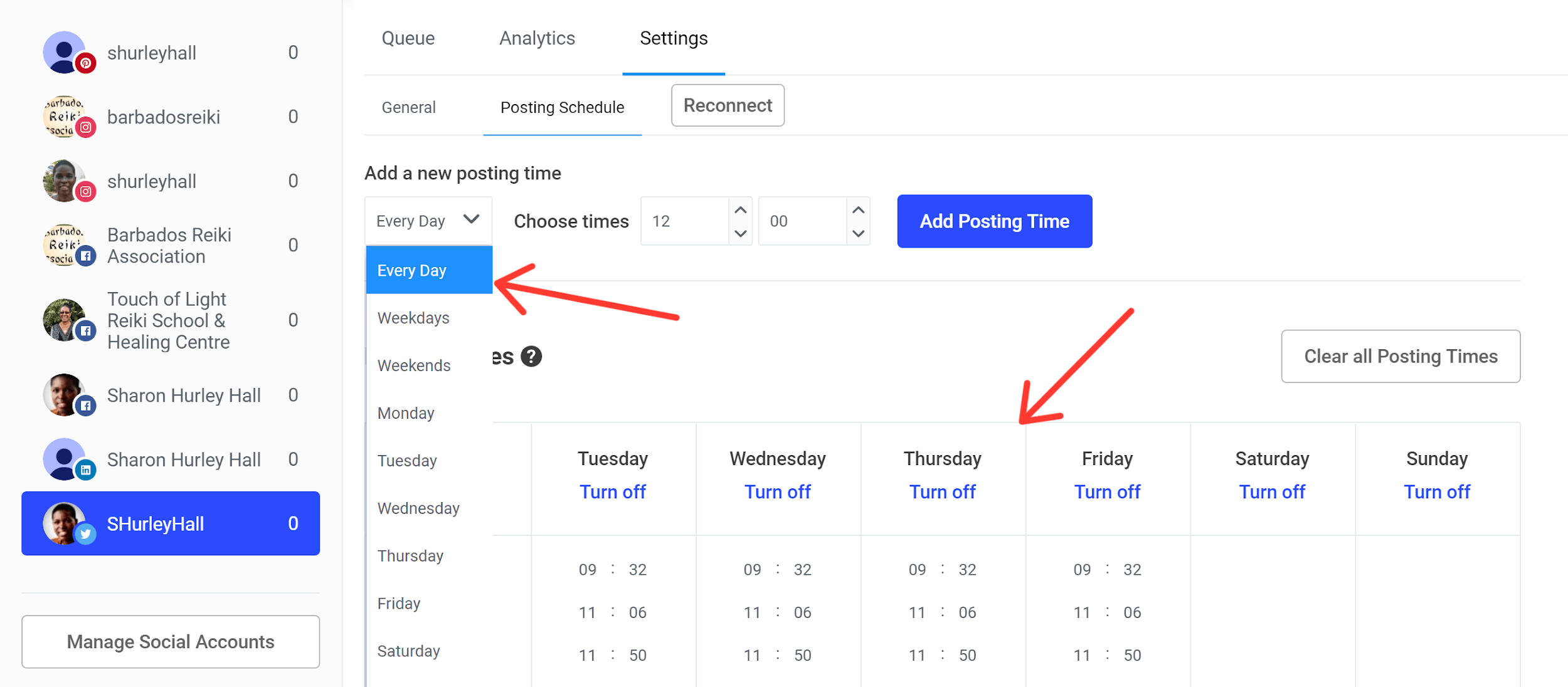
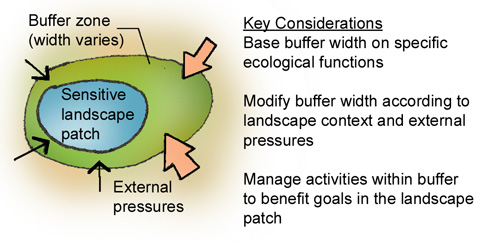
Buffer, pH control, acid-base balance, buffer solutions
Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. Ions are atoms or molecules that have lost or gained one or more electrons. An example of a common buffer is a solution of acetic acid (CH3COOH) and sodium
Why does a higher concentration of weak acid improve a buffer solutions ability to neutralise acids? - Quora

Buffering and Henderson-Hasselbalch equation: Video

Brilliant buffers, Feature
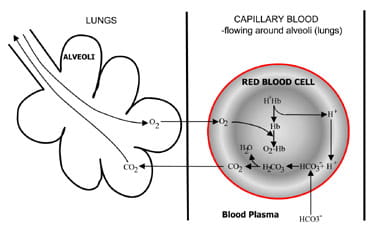
An introduction to acid-base balance in health and disease

Acid base balance

The Role of Buffers in Establishing a Balance of Homeostasis and Maintaining Health, American Journal of Medicinal Chemistry, Science Repository
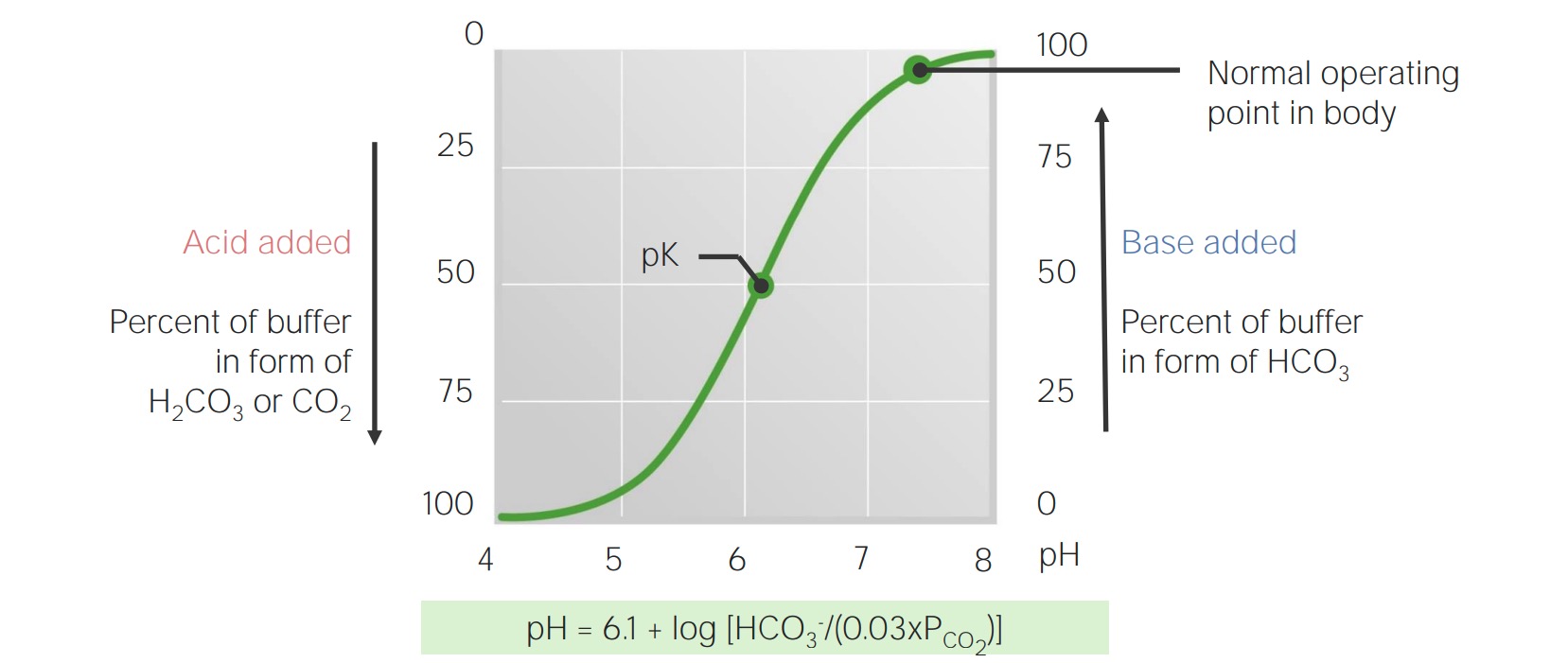
Acid-Base Balance Concise Medical Knowledge

SOLUTION: Clinical chemistry acid base balance worksheet 2 - Studypool

Chemistry of buffers and buffers in our blood (article)

How the Kidneys Regulate Acid Base Balance - Video & Lesson
20 Fascinating Facts About Blood Buffer
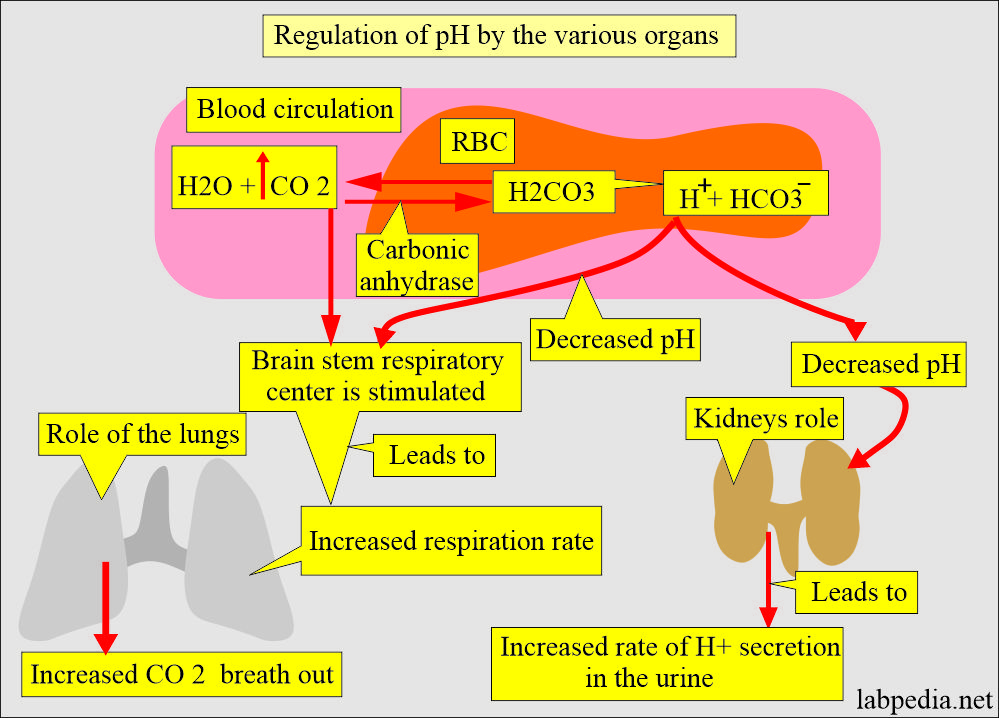
Acid-base Balance:- Part 1 A - Introduction to the Acid-Base Balance

pH Buffers & pH Solutions - Comprehensive Guide – Spectra Scientific