Using Dermabond for Wound Closure in Lumbar and Cervical Neurosurgical Procedures
This study demonstrates that Dermabond is safe to use in neurosurgery patients undergoing lumbar or cervical procedures, with only 1 patient of 200 having a proven infection. OBJECTIVE: 2-Octylcyanoacrylate (Dermabond; Ethicon, Inc., Somerville, NJ) is a liquid adhesive being used with increasing frequency for the closure of lacerations and surgical incisions. Dermabond provides excellent cosmetic closure, and recent studies have demonstrated very low infection risks when it is properly applied. There are no published studies using Dermabond on lumbar or cervical procedures. This study was undertaken to determine whether Dermabond is safe and efficacious to use in these common neurosurgical procedures. METHODS: Records of 200 consecutive patients with Dermabond closure after anterior cervical discectomy, microlumbar discectomy, or lumbar laminectomy by the senior author (JEB) with a mean follow-up time of 5.42 months were retrospectively reviewed. Suspected infections with or without confirmatory cultures, erythema, and incisional drainage were documented. RESULTS: Of 200 patients, 85 underwent microlumbar discectomy, 22 lumbar laminectomy, and 93 anterior cervical discectomy. There was only 1 definitive infection, which was a culture-proven discitis in a microlumbar discectomy patient. Of the remaining 85 microlumbar discectomies, there was 1 transient incisional erythema. Of the 22 lumbar laminectomies, there was 1 patient with clinical superficial wound infection with negative cultures and 4 patients with transient incisional drainage without infection. Of the 93 anterior cervical discectomies, 2 had transient incisional drainage without infection. CONCLUSION: This study demonstrates that Dermabond is safe to use in neurosurgery patients undergoing lumbar or cervical procedures, with only 1 patient of 200 having a proven infection. Patients are able to shower and do not have sutures or staples to remove. Patient responses are overwhelmingly positive.

2-Octyl Cyanoacrylate Skin Adhesive for Topical Skin Incision Closure in Female Pelvic Surgery

Cyanoacrylate Dermal Closure in Spine Surgery: Systematic Review and Pooled Analysis - Terence Tan, Joost Rutges, Travis Marion, Martin Hunn, Jin Tee, 2020

Month __, 20__. TOPICAL SKIN ADHESIVES DERMABOND ® Topical Skin Adhesive Product 1 Product ppt download

PDF) Superiority of octyl-2 cyanoacrylate over polyamide black for surgical site incisions. Prospective randomized trial

Ureasil–polyether hybrid film-forming materials - ScienceDirect

The cyanoacrylate topical skin adhesives - ScienceDirect

Intrathecal Pump Implantation: Technique and Precautions
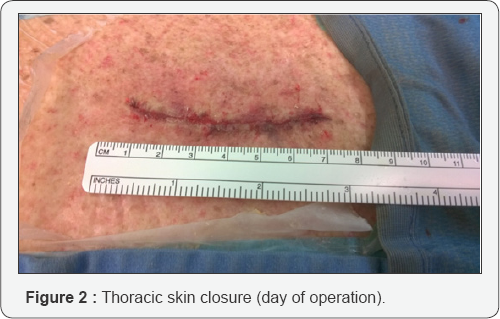
Open Access Journal of Neurology & Neurosurgery
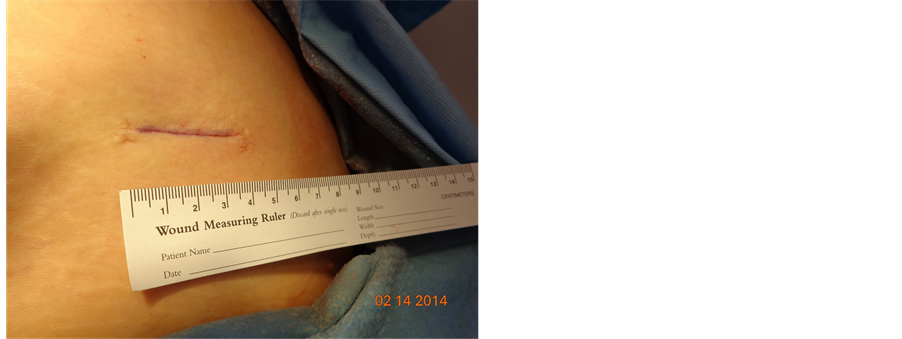
2-Octyl Cyanoacrylate Skin Adhesive for Topical Skin Incision Closure in Female Pelvic Surgery

Spine surgery vaccaro by Neurocirurgiao bh - Dr Eric Grossi - Issuu

A comparison of 2-octyl cyanoacrylate with nylon for wound closure of knee arthroscopy portals