
Characterization and Stability Study of Polysorbate 20 in

Pharmaceutics, Free Full-Text

PDF) Study of the reaction of polysorbate-20 with heteropolyacids

Formulation mitigations for particle formation induced by enzymatic hydrolysis of polysorbate 20 in protein-based drug products: insights from a full-factorial longitudinal study, AAPS Open

Mixed-mode chromatography in pharmaceutical and biopharmaceutical

Effect of Polysorbate 20 and Polysorbate 80 on the Higher-Order

Characterization and Stability Study of Polysorbate 20 in Therapeutic Monoclonal Antibody Formulation by Multidimensional Ultrahigh-Performance Liquid Chromatography–Charged Aerosol Detection–Mass Spectrometry

Residual Host Cell Protein Promotes Polysorbate 20 Degradation in a Sulfatase Drug Product Leading to Free Fatty Acid Particles - Journal of Pharmaceutical Sciences

Intra-Micellar and Extra-Micellar Oxidation in Phosphate and Histidine Buffers Containing Polysorbate 80 - Journal of Pharmaceutical Sciences
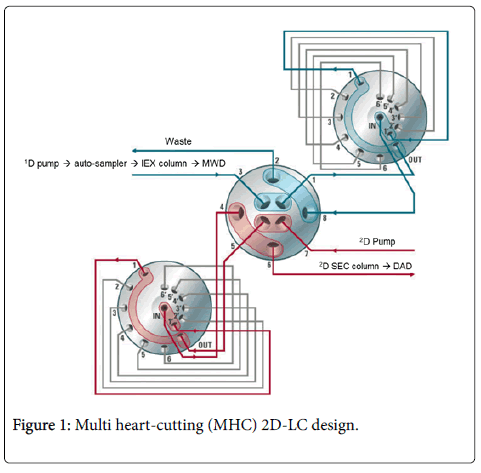
Forced Degradation Study of Monoclonal Antibody Using Two-Dimensi

All-in-one stability indicating polysorbate 20 degradation root

Development and validation of a selective marker-based quantification of polysorbate 20 in biopharmaceutical formulations using UPLC QDa detection - ScienceDirect
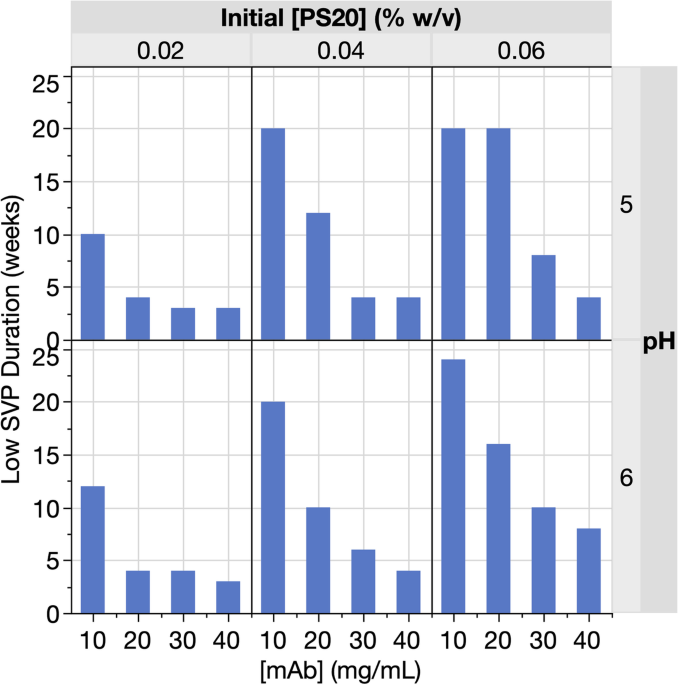
Formulation mitigations for particle formation induced by enzymatic

The Characterization of Polysorbate - Zhang - 2022 - Current

Simultaneous quantification of polysorbate 20 and poloxamer 188 in biopharmaceutical formulations using evaporative light scattering detection - ScienceDirect

Challenges in polysorbate characterization by mass spectrometry









