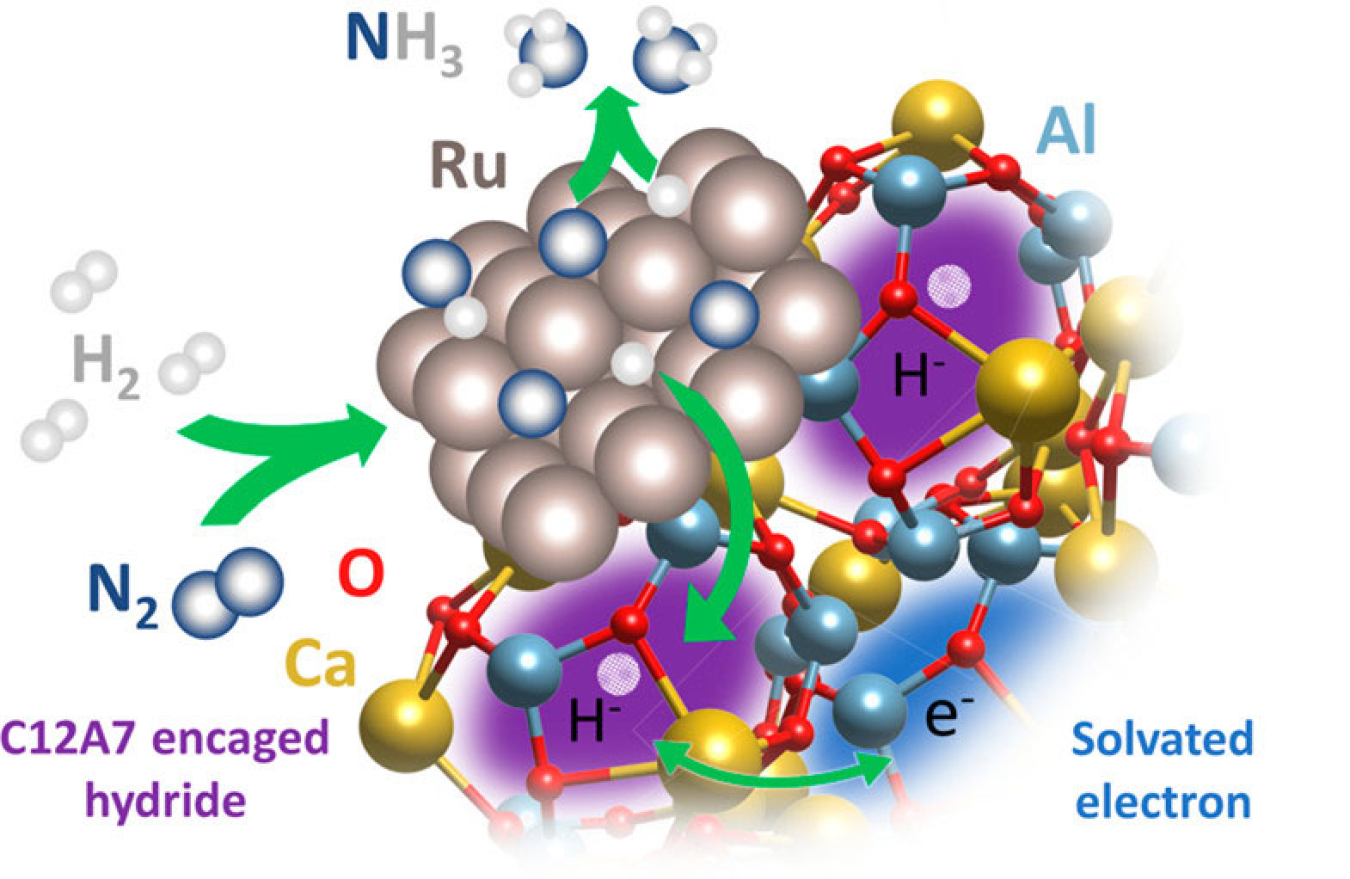
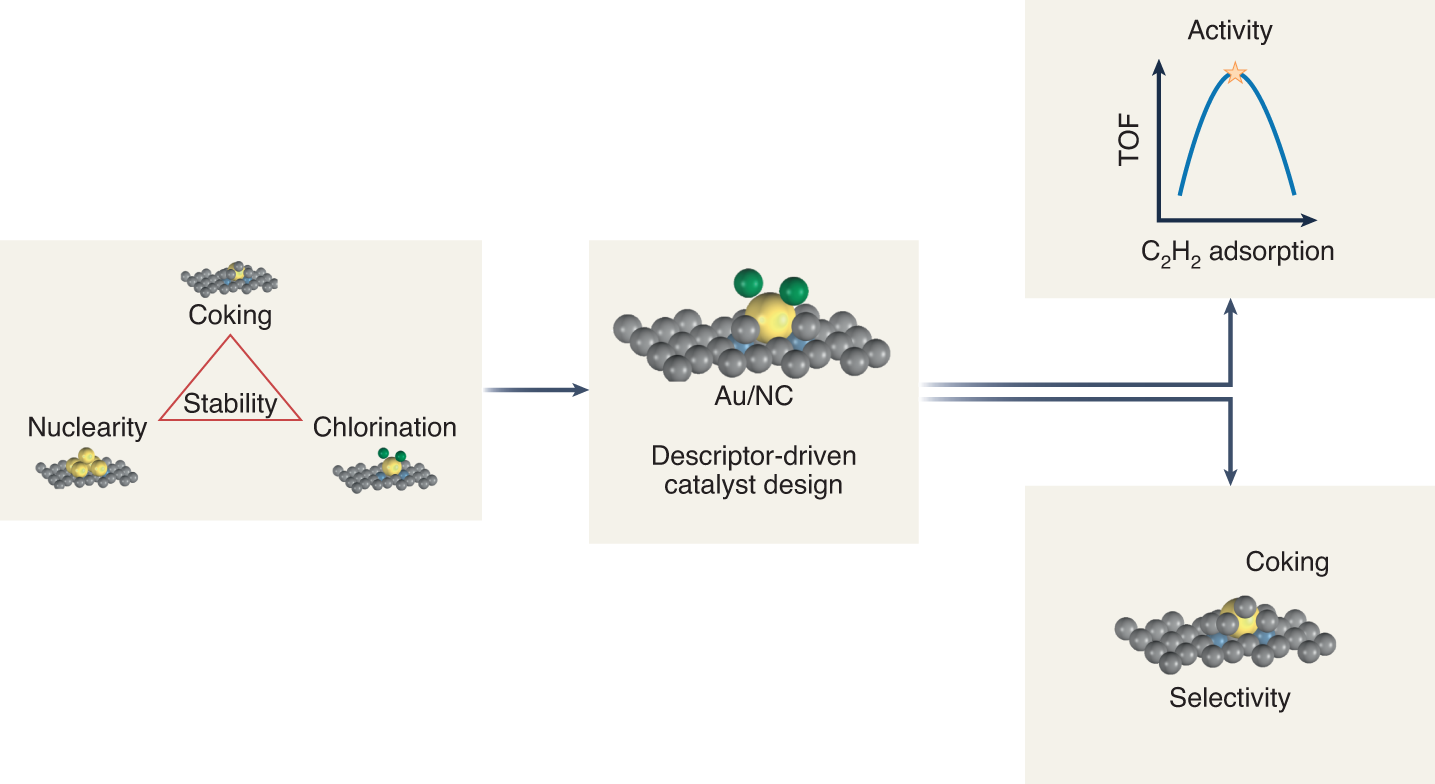
Substrate, Catalyst, and Solvent: The Triune Nature of Multitasking Reagents in Hydroboration and Cyanosilylation
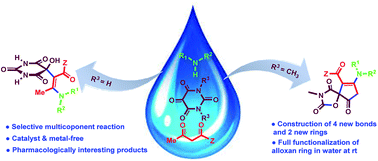
Substrate-controlled selectivity switch in a three-component reaction: sequential synthesis of spiro-oxazolidinedione-cyclopentenones and hydroxy enaminobarbiturates in water - RSC Advances (RSC Publishing)

Highly Enantioselective Cyanosilylation of Aldehydes Catalyzed by

A Facile and Green Pathway for One‐Pot Multicomponent Synthesis of Functionalized Spiroxyindoles using Caffeinium Hydrogen Sulfate as a Catalyst. - Agarwal - 2019 - ChemistrySelect - Wiley Online Library

Sakya SEN, Professor (Assistant), PhD, CSIR - National Chemical Laboratory, Pune, Pune, NCL, Catalysis Division (NCL)

Sandeep YADAV, JRF

Catalyst-free one-pot cascade cyclization: An efficient synthesis of isoindolobenzoxazinones and isoindoloquinazolinones derivatives - ScienceDirect

Catalyst-Free Approach for Hydroboration of Carboxylic Acids under Mild Conditions

Aluminum-Catalyzed Hydroboration of Alkenes

The Metal−Ligand Bifunctional Catalysis: A Theoretical Study on

Theoretical Insights into Aluminum-Catalyzed Cyanosilylation of

Non-enzymatic catalytic asymmetric cyanation of acylsilanes
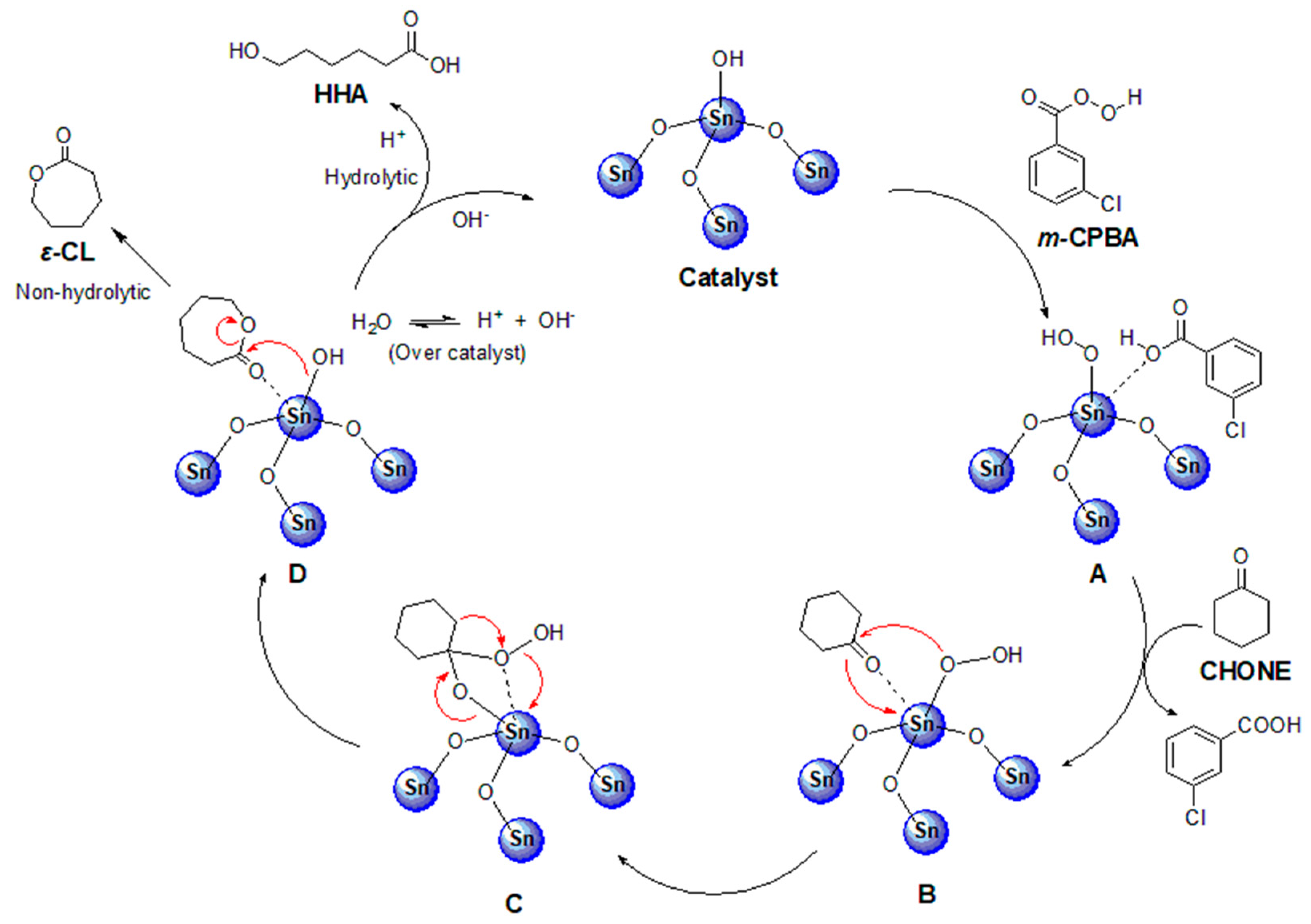
Catalysts, Free Full-Text

Enantioselective Copper-Catalyzed Cyanation of Remote C(sp3)-H Bonds Enabled by 1,5-Hydrogen Atom Transfer - ScienceDirect