
Dry ice, Sublimation, Temperature, Uses
Dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °C (−109.3 °F), used as a refrigerant, especially during shipping of perishable products such as meats or ice cream. In the production of dry ice
Why is dry ice solid at room temperature whereas CO2 is gas? - Quora
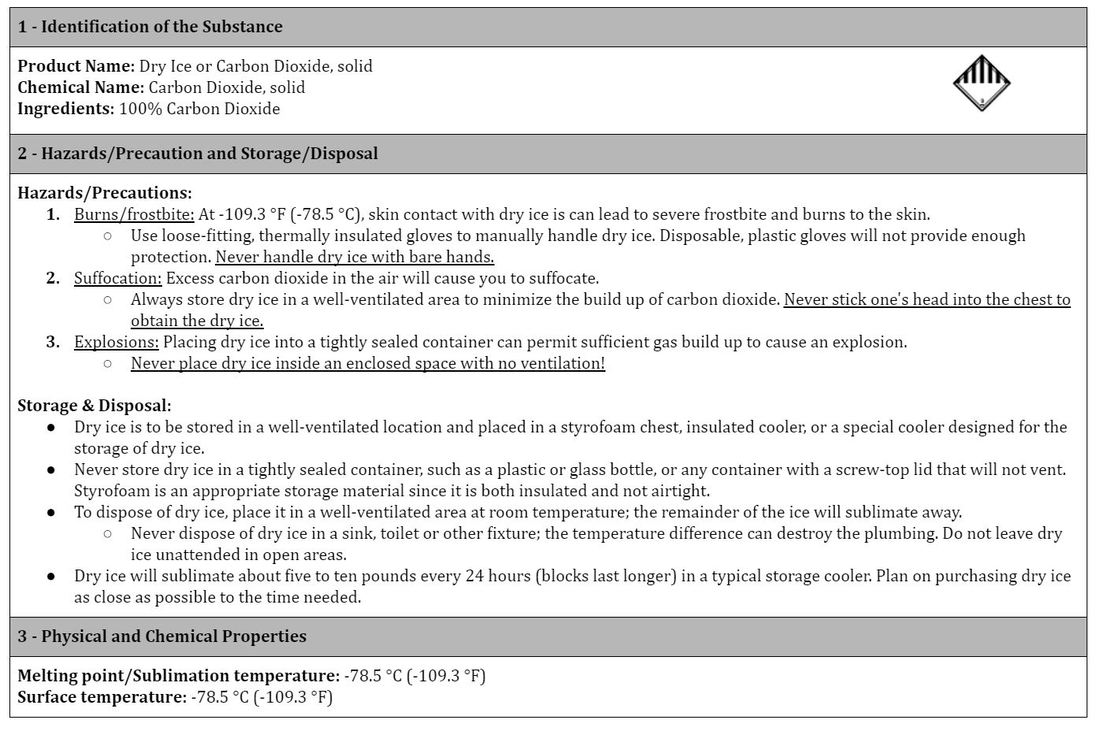
Using Dry Ice? - Gretchen Brinza

Dry Ice Exploration (FREE Printable Included!)
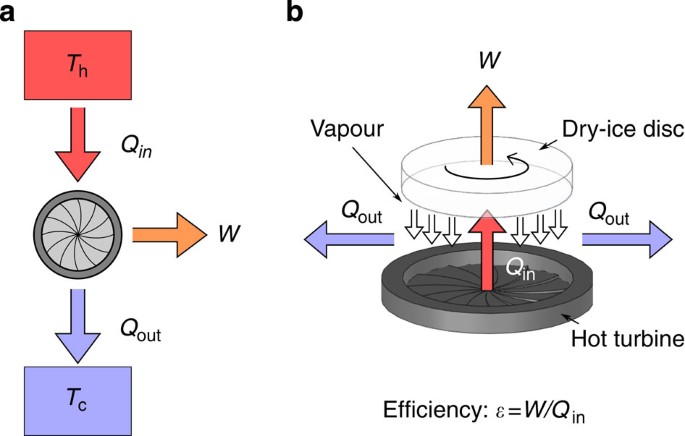
A sublimation heat engine
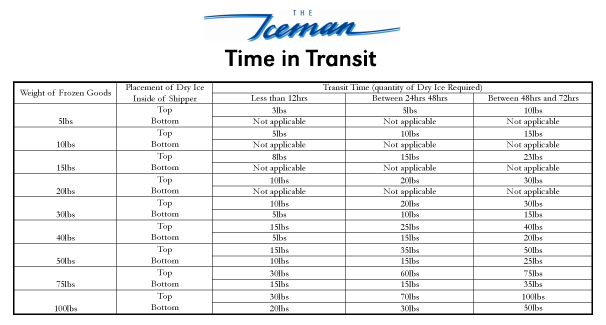
Dry Ice Calculator – Iceman Toronto

Solved Dry ice (solid carbon dioxide) sublimes at the phase

Dry Ice Background Dry ice is carbon dioxide in its solid state. Remember…dry ice sublimates instead of melting. Carbon dioxide is found in the earth's. - ppt download

Dry ice, CO_2(s), does not melt at atmospheric pressure. It sublimes at a temperature of -78 degrees Celsius. What is the lowest pressure at which CO_2(s) will melt to give CO_2(l)?

How Long Does Dry Ice Last? In-Depth Look at Shelf Life, Storage, and Expiration

Cooling an Environmental Chamber with Dry Ice. What's the coldest you can get the air temp down to? - Mechanical engineering general discussion - Eng-Tips

Experimental and theoretical investigation of the dry ice sublimation temperature for varying far-field pressure and CO2 concentration - ScienceDirect









