Using Dermabond for Wound Closure in Lumbar and Cervical
This study demonstrates that Dermabond is safe to use in neurosurgery patients undergoing lumbar or cervical procedures, with only 1 patient of 200 having a proven infection. OBJECTIVE: 2-Octylcyanoacrylate (Dermabond; Ethicon, Inc., Somerville, NJ) is a liquid adhesive being used with increasing frequency for the closure of lacerations and surgical incisions. Dermabond provides excellent cosmetic closure, and recent studies have demonstrated very low infection risks when it is properly applied. There are no published studies using Dermabond on lumbar or cervical procedures. This study was undertaken to determine whether Dermabond is safe and efficacious to use in these common neurosurgical procedures. METHODS: Records of 200 consecutive patients with Dermabond closure after anterior cervical discectomy, microlumbar discectomy, or lumbar laminectomy by the senior author (JEB) with a mean follow-up time of 5.42 months were retrospectively reviewed. Suspected infections with or without confirmatory cultures, erythema, and incisional drainage were documented. RESULTS: Of 200 patients, 85 underwent microlumbar discectomy, 22 lumbar laminectomy, and 93 anterior cervical discectomy. There was only 1 definitive infection, which was a culture-proven discitis in a microlumbar discectomy patient. Of the remaining 85 microlumbar discectomies, there was 1 transient incisional erythema. Of the 22 lumbar laminectomies, there was 1 patient with clinical superficial wound infection with negative cultures and 4 patients with transient incisional drainage without infection. Of the 93 anterior cervical discectomies, 2 had transient incisional drainage without infection. CONCLUSION: This study demonstrates that Dermabond is safe to use in neurosurgery patients undergoing lumbar or cervical procedures, with only 1 patient of 200 having a proven infection. Patients are able to shower and do not have sutures or staples to remove. Patient responses are overwhelmingly positive.

PDF) Wound closure with a mesh and liquid tissue adhesive

The cyanoacrylate topical skin adhesives - ScienceDirect

In Vitro Assessment of Microbial Barrier Properties of Dermabond® Topical Skin Adhesive

A comparison of 2-octyl cyanoacrylate with nylon for wound closure of knee arthroscopy portals

Using Dermabond for Wound Closure in Lumbar and Cervical Neurosurgical Procedures
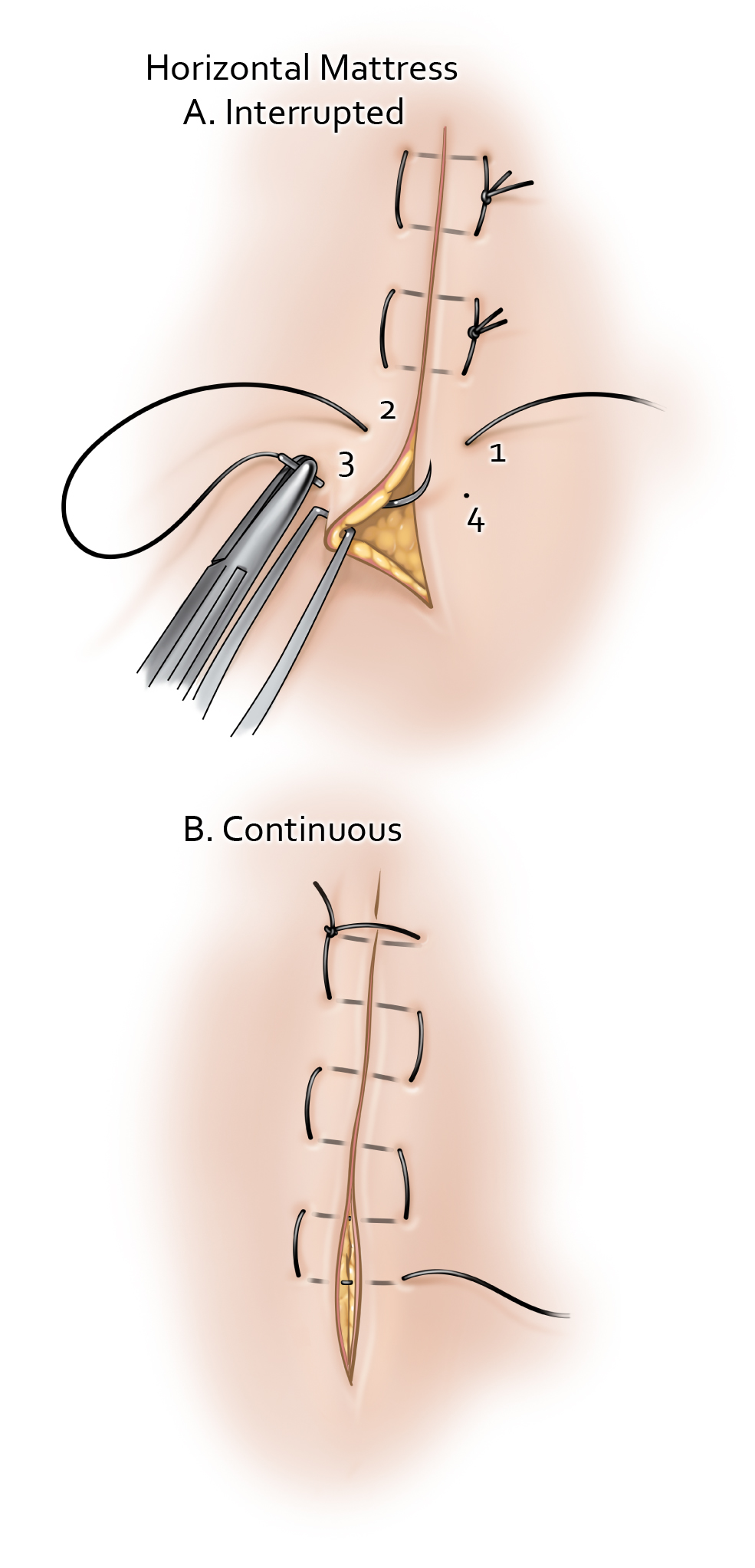
Suturing and Closure The Neurosurgical Atlas
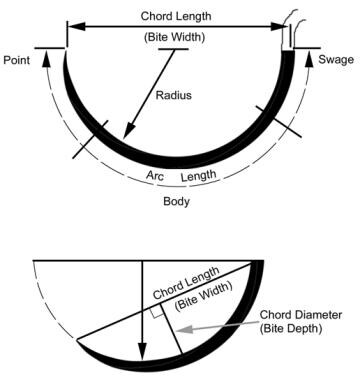
Materials for Wound Closure: Wound Healing and Closure, Suture

Allergic contact dermatitis to Dermabond Prineo after abdominal
Use of 2-Octyl Cyanoacrylate Together with a Self-Adhering Mesh

Development and characterization of film forming polymeric solutions for skin drug delivery - ScienceDirect

2-Octyl-cyanoacrylate for wound closure in cervical and lumbar spinal surgery