14.6: Buffers - Chemistry LibreTexts
A solution containing a mixture of an acid and its conjugate base, or of a base and its conjugate acid, is called a buffer solution. Unlike in the case of an acid, base, or salt solution, the …
A solution containing a mixture of an acid and its conjugate base, or of a base and its conjugate acid, is called a buffer solution. Unlike in the case of an acid, base, or salt solution, the hydronium ion concentration of a buffer solution does not change greatly when a small amount of acid or base is added to the buffer solution. The base (or acid) in the buffer reacts with the added acid (or base).

5.1: Day 36- Buffer Solutions - Chemistry LibreTexts

PPAR-LAB-PRESENTATION-2.pptx - PHYSICAL PHARMACY LABORATORY ASSIGNMENT 2 BUFFERS & ISOTONIC SOLUTION Q U E S TI O N S 1.What is HLB? Write any

Buffers - Chemistry LibreTexts, PDF, Buffer Solution
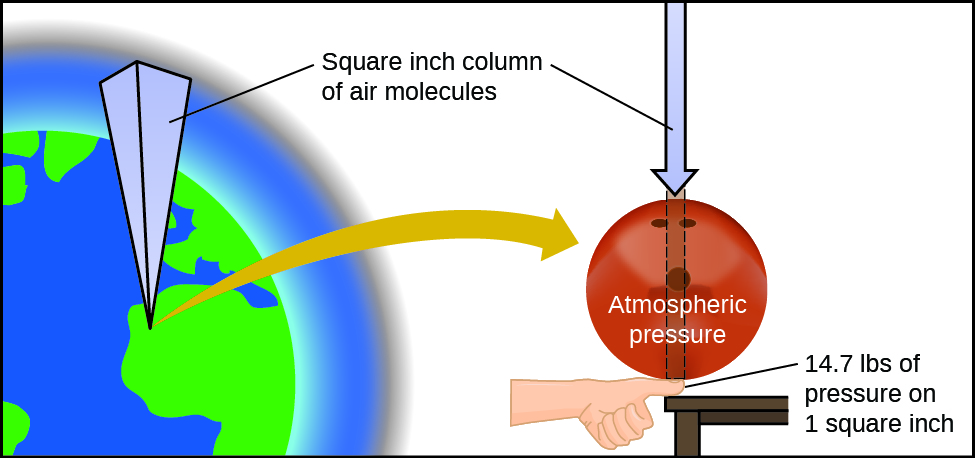
12.1 Gas Pressure – Enhanced Introductory College Chemistry
16.7 Buffers – Chemistry v. 1 backup
14.5: Polyprotic Acids - Chemistry LibreTexts

Scientific Explanation Of Reaction Lab Answers

Buffers - Chemistry LibreTexts, PDF, Buffer Solution

Surface immobilization strategies for the development of
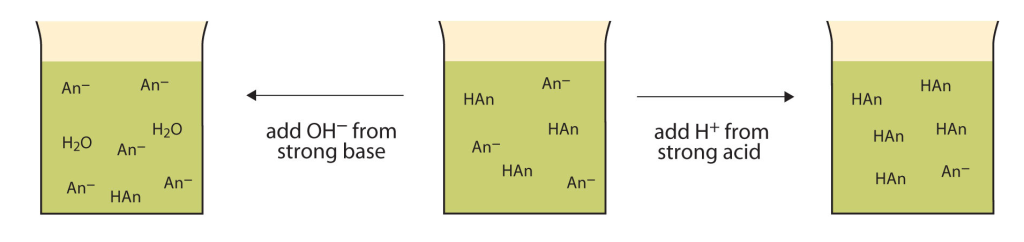
16.7 Buffers – Enhanced Introductory College Chemistry
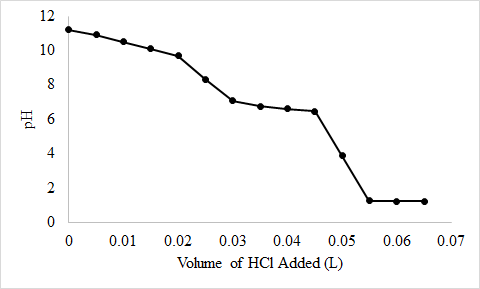
4.E: Buffer, Solubility, Common Ion Effects, and Acid-Base

Direct Immersion–Solid-Phase Microextraction Coupled to Gas
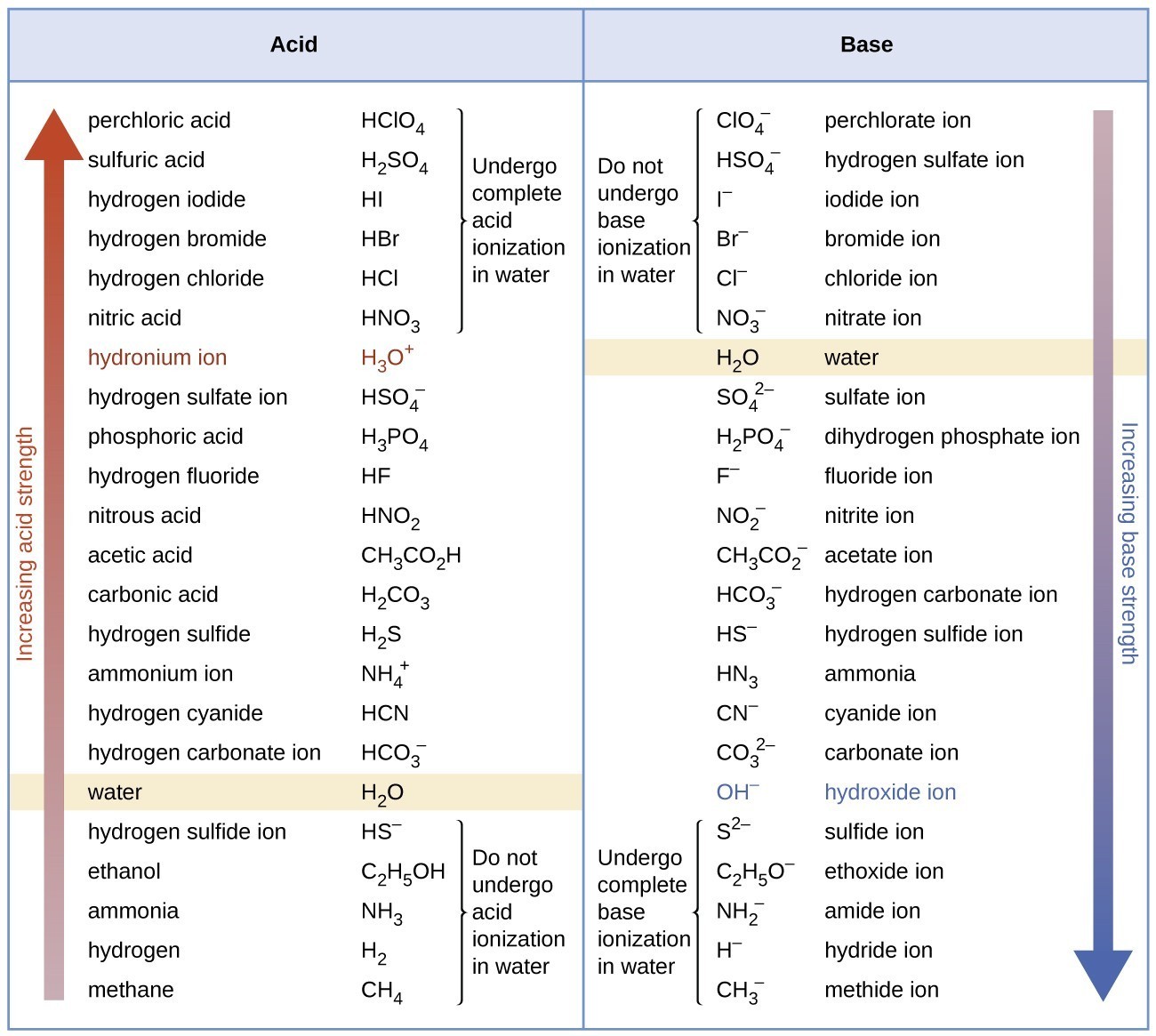
14.3: Relative Strengths of Acids and Bases - Chemistry LibreTexts
Introduction to Chemistry - Chemistry LibreTexts
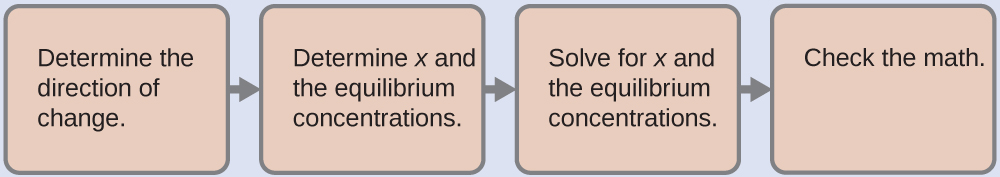
14.6: Buffers - Chemistry LibreTexts